The term molarity is mostly associated with Chemistry. It is referred to as the measure of the concentration of a chemical compound found in a solute. A solute is a solid that dissolves into a liquid to form a solution. Molarity is expressed in terms of the amount of substance per unit volume of a solution.
The question of calculating molarity is common in chemistry examinations. The question that most students have is whether or not such concepts in Chemistry are applied in other situations. However, molarity applies to everyday functions. For instance, when you add sugar to water when making tea. This counts as making a solution. The measure of the concentration of chemical components found in sugar in the mixture counts as molarity. This does not count as a regular equation of molarity, but it is not far from the molarity that we calculate in Chemistry.
Definition of important terms as used in calculating molarity
- Concentration- In Chemistry, the word concentration refers to the abundance of a constituent when it is divided by the total volume of a mixture. The term can be used in any type of mixture. However, it is more commonly used in the case of solutes and solvents.
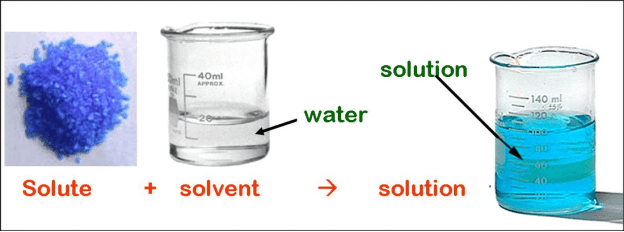
- Solute- a solute is a substance that is dissolved in a given solution. For instance, in a solution of water and sugar, sugar is the solute because it is dissolved into a liquid.
- Solvent- a solvent is a substance that dissolves a solute hence resulting in a solution. For instance, in the case of water and sugar, water is the solvent. Most of the time, a solvent is a liquid. However, a solvent can also be a gas, solid, or supercritical fluid.
- Solution- a solution can be termed as a type of any homogeneous mixture made up of either 2 or more substances. In the case of molarity, a solution is a mixture of a solute and a solvent.
- Amount of substance- In Chemistry, the amount of substance refers to the number of discrete atomic-scale particles found in a given sample of matter.
- Volume- Volume is the quantity of three-dimensional space that is enclosed within a closed surface or structure like a solid, liquid, or gas. It is also defined as the capacity of a container. It is, therefore, the amount of liquid or gas that can be held inside a certain container. Volume is represented in a cubic meter. However, in liquids, the volume is expressed in liters.
- Mole- Mole, also known as mol, is a unit of measurement for the amount of substance. It can also be defined as the number of atoms in a substance. In Chemistry, the word mole refers to the number of reactants and products of a chemical reaction.
Conclusion
The molarity of a solution is calculated by dividing the number of moles of any solute in any solution by the volume of the solution. It is important to remember that molarity is calculated per liters of solution. Molarity can either be calculated manually or using a molarity calculator tool found on the internet.